No time to read the details? Get my abbreviated CV here in PDF form.
Postdoctoral work
Upon infection of its host, Rift Valley fever virus (RVFV) inhibits transcription by interacting with the mammalian p62 subunit of TFIIH. Hijacking of cellular processes is afforded by the formation of a complex between RVFV NSs protein and p62-TFIIH, which leads to the degradation of the complex and complete arrest of transcription. From an infectious agent perspective, such a dramatic effect procures several advantages, notably the abrogration of transcription of genes involved in the type I interferon response to the viral infection. In the James Omichinski laboratory at Université de Montréal, we use a structural biology approach to characterize the molecular basis for the NSs interaction with the p62 subunit of TFIIH.
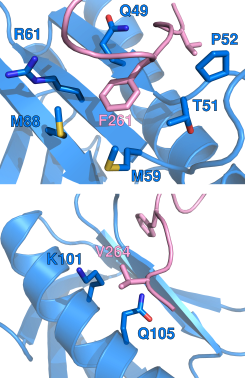
In collaboration with Kylene Kehn-Hall’s group at the National Center for Biodefense and Infectious Diseases (George Mason University, VA USA), our research has identified a short linear motif found at the C-terminus of the virulence factor NSs which is critical for the interaction with the host p62 subunit of TFIIH. Using protein NMR and other biophysical techniques, we have discovered that the interaction mimics the process by which certain nucleotide excision repair factors and transcription factors interact with p62. Further, functional studies in RVFV-infected cells show that the short linear motif is required for both nuclear filament formation and degradation of p62 and mediating the virulence of the pathogen (Cyr et al. 2015).
In a secondary project, we have performed a detailed atomic description of the interactions between the transactivation domain 1 of the p65 subunit of NF-κB and transcription regulatory factors, including the p62 subunit of TFIIH. We identified key hydrophobic residues in the transactivation domain implicated in interactions with transcription regulatory factors and capable of activating gene transcription (Lecoq et al. 2017).
Related publications
- Cyr N, de la Fuente C, Lecoq L, Guendel I, Chabot PR, Kehn-Hall K, Omichinski JG (2015). “A ΩXaV motif in the Rift Valley fever virus NSs protein is essential for degrading p62, forming nuclear filaments and virulence”. Proc Natl Acad Sci USA 112:6021-6026. (abstract), PDB 2N0Y)
- Radio interview on Voice of America Afrique (Carrefour Science et Santé)
- Article in MedicalXpress, Newswise, HealthCanal and UdeM news.
- Lecoq L, Raiola L, Chabot PR, Cyr N, Arseneault G, Legault P, Omichinski JG (2017). “Structural characterization of interactions between transactivation domain 1 of the p65 subunit of NF-κB and transcription regulatory factors”. Nucleic Acids Res, in press. (PDB 2N22 and 5U4K)
PhD work
Leishmania
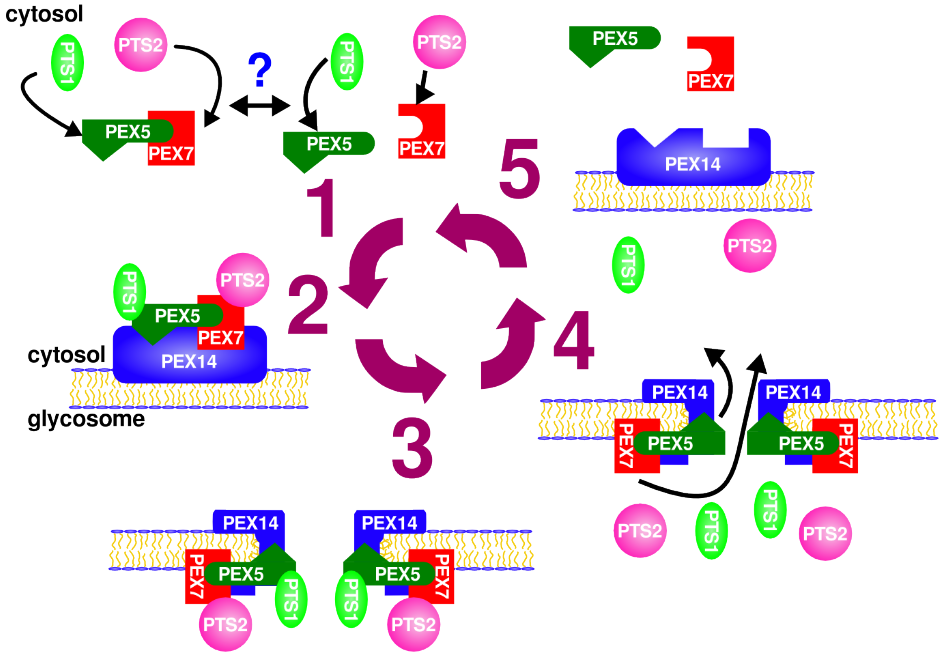
Leishmania, and other kinetoplastids, compartmentalize glycolysis and other vital metabolic pathways in the glycosome - a subcellular organelle that is evolutionary related to peroxisomes. Previous studies have indicated that disturbance in the targeting of proteins to the glycosome results in a lethal phenotype for these parasites. Consequently the glycosome biogenesis machinery consists of an attractive therapeutic target. During my doctoral studies with Dr. Armando Jardim, I uncovered a key component of the glycosomal protein import machinery, L. donovani PEX14, a membrane-associated protein required for translocation of proteins across the glycosomal membrane.
Quaternary structure analysis of LdPEX14 revealed that this protein forms a large oligomeric complex. Domain mapping showed that elimination of a hydrophobic region and a coiled-coil motif were necessary to disrupt oligomer formation (Cyr et al. 2008). Moreover, calorimetry, intrinsic fluorescence, circular dichroism and analytical ultracentrifugation experiments showed that binding of LdPEX5 caused a dramatic conformational change in the LdPEX14 complex that was accompanied by a reorganization of a hydrophobic segment, common to other PEX14 proteins (Cyr et al. 2008). These studies provide a more fundamental understanding of the glycosome biogenesis machinery and its interaction with the glycosomal membrane (Pilar 2014, Cyr 2013).
Type III secretion system of enteropathogenic E. coli
I was also involved in a secondary project during my PhD which involved the assembly of the Type III secretion system in the enteropathogenic E. coli. A biophysical approach helped elucidate the interaction between the bacterial protein EspD and the phospholipids constituting the host plasma membrane (Dasanayake et al. 2011).
Related publications
-
Pilar AV, Strasser R, McLean J, Quinn E, Cyr N, Hojjat H, Kottarampatel AH, Jardim A (2014). “Analysis of the Leishmania peroxin 7 interactions with peroxin 5, peroxin 14 and PTS2 ligands”. Biochem J 460:273-282. (abstract)
-
Dasanayake D, Richaud M, Cyr N, Caballero-Franco C, Pitroff S, Finn RM, Ausió J, Luo W, Donnenberg MS, Jardim A (2011). “The N-terminal amphipathic region of the Escherichia coli type III secretion system protein EspD is required for membrane insertion and effacement activity”. Mol Microbiol 81:734-750. (abstract)
-
Ullman B, Cyr N, Choi K, Jardim A (2010). “Acidic residues in the purine binding site govern the 6-oxopurine specificity of the Leishmania donovani xanthine phosphoribosyltransferase”. Int J Biochem Cell Biol 42:253-262. (abstract)
-
Cyr N, Madrid KP, Strasser R, Aurousseau M, Finn R, Ausio J, Jardim A (2008). “L. donovani PEX14 undergoes a marked conformational change following association with PEX5”. J Biol Chem 283:31488-31499. (abstract)
PhD Thesis
- Cyr N (2013). Role of Leishmania donovani peroxin 14 in glycosomal import machinery. Ph.D. thesis, Institute of Parasitology, McGill University. (abstract) (PDF - 47.2 MB)
Previous work
My M.Sc. work was done under the supervision of Prof. John Sheppard in the department of Bioresouce Engineering at McGill University. My project involved the investigation of various bioprocess parameters and their effect on the formation of metabolic by-products during fermentation. We investigated:
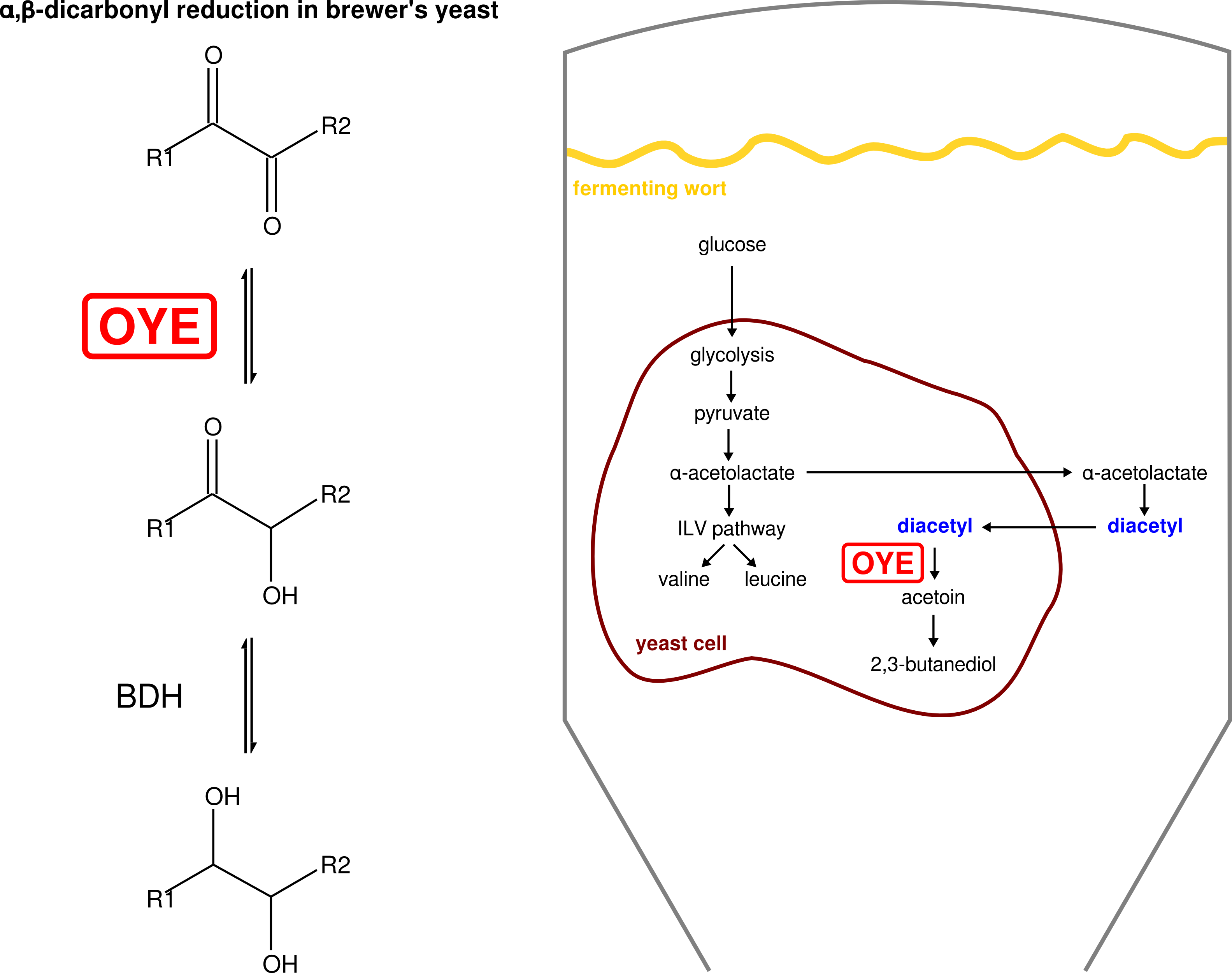
- Putative enzymes involved in the reduction of alpha,beta-dicarbonyl compounds by Saccharomyces cerevisiae (van Bergen, Cyr el al. 2016; van Bergen et al. 2006)
-
Usage of industrial drying processes in the manufacturing of ale and lager Brewer’s dry yeast and their implication in amino acid metabolism and vicinal diketone formation profile in Saccharomyces during industrial beer fermentation (Cyr et al. 2007).
-
Effect of various aeration strategies on metabolic by-products in very high gravity continuous fuel ethanol fermentation processes (Cyr 2006).
Related publications
-
van Bergen B, Cyr N*, Strasser R, Blanchette, M, Sheppard JD, Jardim A (2016). “α,β-Dicarbonyl reduction is mediated by the *Saccharomyces Old Yellow Enzyme”. FEMS Yeast Res 16:fow059. (* equal contribution) (abstract)
-
Cyr N, Blanchette M, Price SP and Sheppard, JD (2007). “Vicinal diketone production and amino acid uptake by two active dry lager yeasts during beer fermentation”. J Am Soc Brew Chem 65:138-144. (abstract)
-
van Bergen B, Strasser R, Cyr N, Sheppard JD, Jardim A (2006). “α,β-Dicarbonyl reduction by Saccharomyces D-arabinose dehydrogenase”. Biochim Biophys Acta 1760:1636-1645. (abstract)
MSc thesis
- Cyr N (2006). Effect of aeration strategy on the performance of a very high gravity continuous fuel ethanol fermentation process. M.Sc. thesis, Department of Bioresource Engineering, McGill University. (abstract) (PDF - 3.8 MB)
Other scientific publications
- van Zandycke S, Ahlgren S, Bourdaudhui G, Cyr N, De Nicola R, Fujita A, Kelly G, Logsdon D, McLean D, Pachello C (2005). “Method for measurement of yeast vitality”. J Am Soc Brew Chem 63:205-208.